Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
A macrocyclic peptide kills bacteria by blocking lipid transport
Drug candidate blocks lipid transport in high-priority resistant pathogen
by Laurel Oldach
March 19, 2024

Finding new antibiotics is a matter of increasing public health concern as drug-resistant bacteria spread around the world. But the task is notoriously difficult; on top of the challenge of developing safe, effective drugs, new antibiotics must be used sparingly to prevent resistance, slowing sales. This week, researchers at Roche are reporting an antibiotic drug candidate that works by a new mechanism to target a single pathogen, and appears to be well tolerated in humans (Nature 2024, DOI: 10.1038/s41586-023-06873-0 and 10.1038/s41586-023-06799-7). The antibiotic targets one of the World Health Organization’s highest-priority pathogens, carbapenem-resistant strains of Acinetobacter baumannii, which can be highly deadly. In an email, Monique van Hoek, director of George Mason University’s Center for Infectious Disease Research, writes that the new molecule “show[s] great potential in treating this challenging organism.”
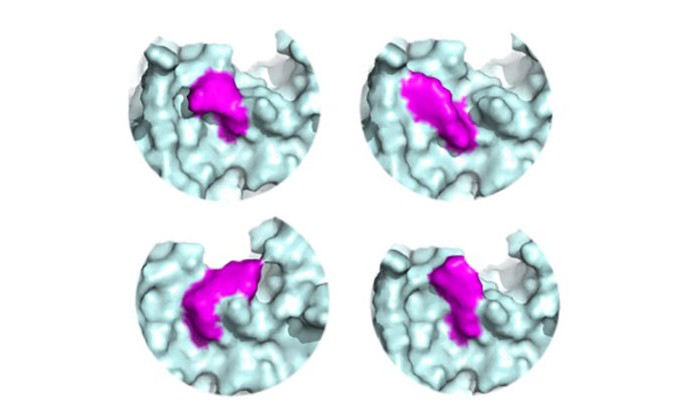
Roche scientists identified the molecule, a macrocyclic peptide dubbed zosurabalpin, after a screen of 45,000 macrocyclic peptides found a few that killed A. baumannii and other double-membraned bacteria without harming human cells. The initial candidate molecule cross-reacted with lipids in mouse plasma, so the researchers optimized to a zwitterionic form, with a balance between positively- and negatively-charged functional groups, to reduce its reactivity.
Researchers determined through genetic and structural studies that it works by blocking transport of a lipid called lipopolysaccharide into place in the outer membrane. Because the lipid-shuffling protein complex differs between bacteria, the drug candidate only works on A. baumannii.
It has been over 50 years since the last antibiotic drug with a wholly new mechanism of action received FDA approval – but this is the second paper in the past year to report a molecule with a new mechanism for specific activity against A. baumannii. A compound called abaucin, identified with the help of artificial intelligence, targets another system that moves lipid-modified proteins from inner to outer membrane (Nat. Chem. Biol. 2023, DOI: 10.1038/s41589-023-01349-8). According to Jonathan Stokes, a biochemist at McMaster University and senior author on the paper describing abaucin, precision antibiotics that target individual species may be less likely to evoke widespread resistance than broad-spectrum molecules that stress all of the bacteria in the microbiome at once.
Roche has already completed the first clinical trials of zosurabalpin (Open Forum Infect. Dis. 2023, DOI: 10.1093/ofid/ofad500.1749), and has reported that it is safe and well tolerated in healthy adults. Paul Hergenrother of the University of Illinois Urbana-Champaign, who authored a commentary on the two studies in Nature, tells C&EN that the successful safety study makes zosurabalpin seem more promising than the average new antibiotic candidate.
He says that it’s encouraging to see large companies like Roche investing in the antibiotic resistance challenge. Even if zosurabalpin proves to be safe and effective, however, “the inevitability of resistance is true for this [molecule] as well,” Hergenrother adds. “One needs a steady supply and resupply of antibiotics.”

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter